- Date:
As the world continues to battle the pandemic, companies have rushed to develop an effective vaccine against the virus. On 9 November, Pfizer and BioNTech announced their candidate vaccine exhibited over 90% effectiveness. One week later, Moderna announced theirs displayed an effectiveness of over 94%, increasing hopes that a solution to the pandemic could be within reach. In this edition of Infocus, Joaquin Thul looks at the factors affecting the development and distribution of a vaccine against Covid-19.
According to the Global Alliance for Vaccines and Immunisation, there are currently almost 200 projects seeking to develop a vaccine against Covid-19. At the moment 21 of them are in Phase 1, undergoing small-scale safety trials on humans; 13 projects are in Phase 2, with trials on hundreds of people to assess safety and correct dosage; and 10 of them are in Phase 3, which involve tests on thousands of individuals to further confirm safety and analyse potential side effects. No vaccines have yet been approved for general use (see Figure 1).1
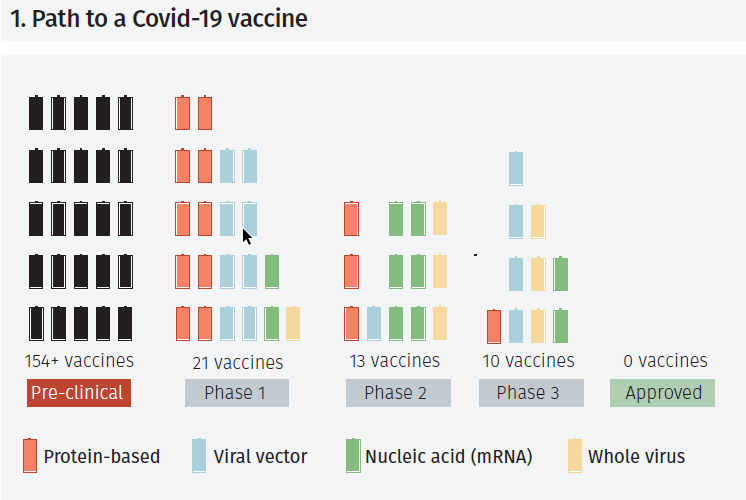
From the projects currently in Phase 3, two of them use a method based on messenger RNA (mRNA). This technique uses synthetic strands of RNA that produce neutralising antibodies once they are injected into the human body. When compared to traditional vaccines, that rely upon dead or weakened forms of the virus (cultured in eggs), the RNA approach is faster, safer, and minimises the risk of accidental transmission. On November 9th, Pfizer and BioNTech announced preliminary results showing their vaccine delivered a high efficacy rate among trial volunteers. These findings are based on tests on 43,538 volunteers, where half received the real vaccine and half received a dummy vaccine or placebo. At the first interim analysis (94 confirmed cases of Covid-19), the vaccine reduced the likelihood of infection by over 90%.2
The companies said they will apply for Emergency Use Authorization to the US Food and Drugs Administration (FDA) by the end of this month and seek approval from EU authorities before the year end. US-based company Moderna has developed another vaccine using the same technology and on 16 November announced preliminary results showing an effectiveness of over 94%.3 Moderna expects to follow a similar process seeking approval. Initial data from AstraZeneca & Oxford University, Johnson & Johnson and Novavax are expected to be released in Q1 2021.
However, there are still several questions to be answered regarding the vaccine approval, efficacy, and availability for mass distribution to the public.
When would a vaccine be available for general distribution?
As is normally the case with new vaccines, making it available for general distribution is a different challenge to getting the treatment approved. The technology used by Pfizer / BioNTech and Moderna, based on genetic material, has never been approved before for use in virus treatments. However, the former two companies have been working on developing a flu vaccine using this same technology since 2018. Although the technology based on mRNA is widely used for cancer treatments, helping immune systems recognise and respond to the proteins produced by specific tumours, the research for its use to combat a virus such as SARS-CoV-2 is still at an early stage.
A separate hurdle relates to the manufacturing process. Pfizer has announced it expects to manufacture enough vaccines to immunise between 15 and 20 million people before the end of 2020 and over 1.3 billion doses for global distribution during 2021.4 One advantage that mRNA technology has over other vaccines is its potential for large scale production. According to medical specialists, the manufacture of this type of vaccine could take only a few months rather than the one or two years it normally takes for conventional vaccines.5
Scalability will be crucial to satisfying the high demand: many countries have already placed large orders for this vaccine. Earlier in the year the US bought 100 million doses of the Pfizer vaccine which they expect to be delivered by December 2020, with the option to acquire another 500 million in 2021. Japan has ordered 120 million doses, the EU 200 million doses, the UK 40 million doses and Australia 10 million doses.6
How long will the immunisation last?
Pfizer and BioNTech’s vaccine requires two doses, taken three weeks apart, to achieve immunisation. The vaccine’s 90% efficacy seven days after the second dose is taken, is much higher than for regular flu vaccines, which are estimated to be between 40% and 60% effective. Although these tests were performed on a diverse group of volunteers, further data are needed to test whether its efficacy changes in different age groups and over longer periods of time without any adverse symptoms. Flu vaccines are generally less effective in older people.
Additionally, more data are also needed to answer whether people will need a booster or annual immunisation against Covid-19 in the future. In that case being vaccinated against the coronavirus could become an annual event ahead of the winter season, as is currently the case with flu vaccinations.
How will governments distribute the vaccine? And how fast can it be deployed?
Distribution of the Pfizer and BioNTech vaccine will be a logistical challenge. The vaccine needs to be kept in special freezer boxes to ensure quality during transport to its destination. However, once delivered these vaccines can be stored in medical fridges for up to five days before being administered to people. Moderna’s vaccine has the advantage of not requiring special storage and transport infrastructure.
It is expected that countries will prioritise the most vulnerable groups of the population to get a vaccine first. We estimate the priority population represents approximately 25% of each country’s total population. A back-of-the-envelope estimate of a country’s vaccination capacity can be obtained by extrapolating from its current Covid-19 testing numbers.
Using this information, we obtain an estimate of the number of months it would take to vaccinate the priority population in each country (see Figure 2).
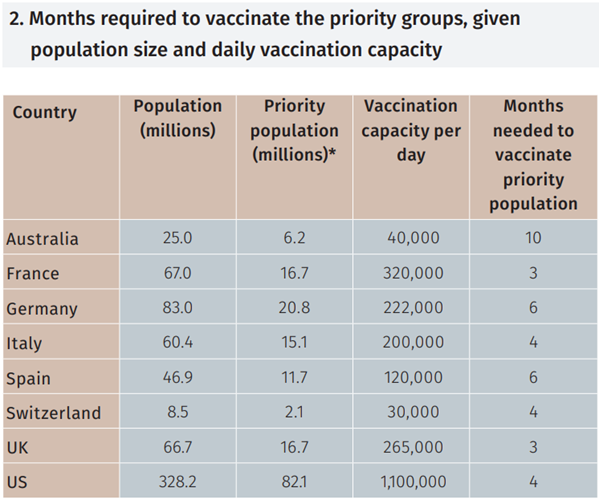
In the UK, the Department of Health has revealed a plan for mass vaccination of its population which is expected to take 10 months and would prioritise the most vulnerable age groups.8 Given the UK population over 60 years old represents approximately 16 million people, it would take over three months to vaccinate the most vulnerable groups. Extending this example to other developed economies we expect to take between three to six months in most countries to provide a vaccine to the over 60s, depending on each country’s vaccination capacity. So the vaccine is not a magic panacea for the pandemic – it will take time for its benefits to be felt.
Regarding its distribution, the World Health Organization has collaborated with governments, private sector companies, scientists and philanthropists to ensure equitable access to vaccines across the world.9 They aim to have two billion doses available by the end of 2021, which should protect high risk and vulnerable cohorts of the population, as well as healthcare workers who are more exposed to the virus. Participating countries will be able to request enough doses to vaccinate between 10%-50% of their populations. However, no country will receive more doses than are enough to vaccinate 20% of its population until all countries in the group have been offered this amount. These vaccines are expected to fall short of the estimated 60% coverage required to achieve herd immunity.10 Currently, 172 countries, representing over 70% of the world’s population, have submitted their interest in participating in this coordinated effort to distribute vaccines globally.11
Conclusions
Initial efficacy results of the newly developed vaccine by Pfizer and BioNTech are extremely promising and represent one of the first steps in overcoming the Covid-19 pandemic. However, the announcement of positive Phase 3 results does not imply by itself that we will immediately be able to return to a normal pre-Covid existence. More testing needs to take place, the treatment needs to be approved in different countries and the vaccine needs to be produced, distributed and administered. Pfizer and BioNTech have stated that although preliminary data has been better than expected, uncertainty remains over the effectiveness of the vaccine in larger or more diverse populations. Additional data and independent assessments are required before this vaccine is ready for commercialisation.12
Even in the case of approval for emergency use, the deployment of this vaccine to higher risk elements of the population will take several months, depending on the capacity of the healthcare systems in each country and the size of the cohort to which the vaccines are to be given. In the meantime, the rest of the population will have to continue adhering to social distancing measures, washing hands regularly and wearing facemasks to protect against the virus.
Nonetheless, despite the short-term challenges, the vaccine represents a major breakthrough in humanity’s fight against Covid-19 and is very much welcome. After a long and difficult period of uncertainty related to the virus, finally there is light at the end of the tunnel.
Footnotes
1 https://www.gavi.org/vaccineswork/covid-19-vaccine-race
2 https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against
3 https://investors.modernatx.com/news-releases/news-release-details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy
4 https://www.nytimes.com/2020/11/09/health/covid-vaccine-pfizer.html
5 https://horizon-magazine.eu/article/five-things-you-need-know-about-mrna-vaccines.html
6 https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
7 https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-longer-shelf-life-its-covid-19-vaccine
8 https://www.bbc.co.uk/news/uk-england-birmingham-54375643
9 https://www.gavi.org/covax-facility
10 https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2020-09-25-COVID19-Report-33.pdf
11 https://www.who.int/news/item/24-08-2020-172-countries-and-multiple-candidate-vaccines-engaged-in-covid-19-vaccine-global-accessfacility#:~:ext=The%2080%20countries%20that%20have,%2C%20Iceland%2C%20Iraq%2C%20Ireland%2C
12 https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against
Important Information
The value of investments and the income derived from them can fall as well as rise, and past performance is no indicator of future performance. Investment products may be subject to investment risks involving, but not limited to, possible loss of all or part of the principal invested.
This document does not constitute and shall not be construed as a prospectus, advertisement, public offering or placement of, nor a recommendation to buy, sell, hold or solicit, any investment, security, other financial instrument or other product or service. It is not intended to be a final representation of the terms and conditions of any investment, security, other financial instrument or other product or service. This document is for general information only and is not intended as investment advice or any other specific recommendation as to any particular course of action or inaction. The information in this document does not take into account the specific investment objectives, financial situation or particular needs of the recipient. You should seek your own professional advice suitable to your particular circumstances prior to making any investment or if you are in doubt as to the information in this document.
Although information in this document has been obtained from sources believed to be reliable, no member of the EFG group represents or warrants its accuracy, and such information may be incomplete or condensed. Any opinions in this document are subject to change without notice. This document may contain personal opinions which do not necessarily reflect the position of any member of the EFG group. To the fullest extent permissible by law, no member of the EFG group shall be responsible for the consequences of any errors or omissions herein, or reliance upon any opinion or statement contained herein, and each member of the EFG group expressly disclaims any liability, including (without limitation) liability for incidental or consequential damages, arising from the same or resulting from any action or inaction on the part of the recipient in reliance on this document.
The availability of this document in any jurisdiction or country may be contrary to local law or regulation and persons who come into possession of this document should inform themselves of and observe any restrictions. This document may not be reproduced, disclosed or distributed (in whole or in part) to any other person without prior written permission from an authorised member of the EFG group.
This document has been produced by EFG Asset Management (UK) Limited for use by the EFG group and the worldwide subsidiaries and affiliates within the EFG group. EFG Asset Management (UK) Limited is authorised and regulated by the UK Financial Conduct Authority, registered no. 7389746. Registered address: EFG Asset Management (UK) Limited, Leconfield House, Curzon Street, London W1J 5JB, United Kingdom, telephone +44 (0)20 7491 9111.



